What Is Cytoplasmic Transfer?
What Is Cytoplasmic Transfer?
What Is Cytoplasmic Transfer?
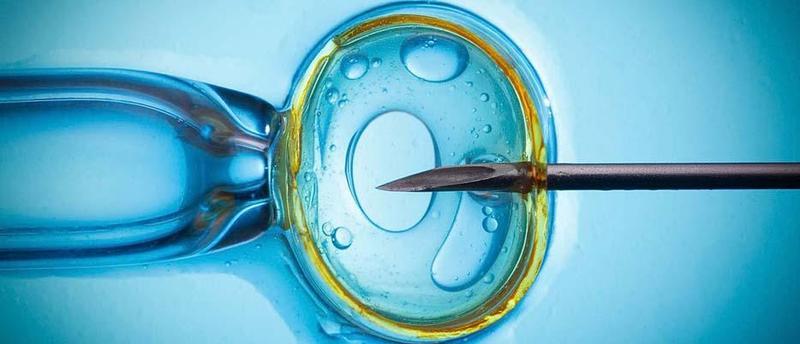
Oplasmic move, otherwise called a cytoplasmic exchange, is a test richness strategy that includes infusing a limited quantity of ooplasm from eggs of ripe ladies into eggs of ladies whose fruitfulness is undermined. The adjusted egg is then treated with sperm and embedded in the uterus of the lady endeavouring to accomplish pregnancy.
Kids conceived from this technique have been accounted for to have cytoplasmic organelles called “ mitochondria” from both their natural mother and the ooplasmic contributor, a condition alluded to as mitochondrial heteroplasmy. Since mitochondria convey their own arrangements of qualities that are given to succeeding ages, the blending of parent and cytoplasmic benefactor mitochondria may be viewed as a type of germline change. In any case, the strategy doesn’t include alteration of specific qualities and couldn’t be utilized as a component of any methodology to make “architect children.”
In 2001, specialists at St. Barnabas Hospital in New Jersey declared that they had utilized ooplasmic move to empower a few ladies with hindered fruitfulness to bear youngsters. They portrayed their work to the press as “the primary instance of human germline alteration.”
Notwithstanding, the ooplasmic move isn’t germline hereditary change, or inheritable hereditary alteration (IGM), in the typical sense. It can’t be utilized to adjust qualities housed in the cell core, which impact all characteristics aside from those controlled by mitochondrial qualities. Yet, the system may be viewed as a type of IGM in that the blended mitochondrial DNA would be given to all people in the future.
Then again, a few backers of IGM have recognized the ooplasmic move as an innovation whose improvement and use could be utilized to help disintegrate mainstream protection from IGM.
Following the St. Barnabas declaration, the FDA informed all US fruitfulness facilities known to offer the strategy that further ooplasm move conventions couldn’t continue without FDA endorsement.
On May 9, 2002, an FDA Advisory Committee held a public gathering to talk about ooplasm move methodology. An assertion gave by the FDA at this gathering announced that at any rate two dozen births credited to ooplasm move have been accounted for by three fruitfulness facilities since 1998.
The FDA communicated worries about this “true germline quality exchange” strategy, referring to its capability to adjust the germline, the clinical dangers related with mitochondrial heteroplasmy, the high frequency of Turner’s condition in embryos revealed in one examination (2 of 13 announced pregnancies), and the lack of creature contemplates and other pre-clinical information. An overall agreement was reached at the gathering that more preclinical information would be essential before FDA would permit further clinical preliminaries including ooplasm move to continue.
Be the first to post a message!