The Neurologist Who Hacked His Brain—And Almost Lost His Mind
The Neurologist Who Hacked His Brain—And Almost Lost His Mind
THE BRAIN SURGERY lasted 11 and a half hours, beginning on the afternoon of June 21, 2014, and stretching into the Caribbean predawn of the next day. In the afternoon, after the anesthesia had worn off, the neurosurgeon came in, removed his wire-frame glasses, and held them up for his bandaged patient to examine. “What are these called?” he asked.
Phil Kennedy stared at the glasses for a moment. Then his gaze drifted up to the ceiling and over to the television. “Uh … uh … ai … aiee,” he stammered after a while, “… aiee … aiee … aiee.”
“It’s OK, take your time,” said the surgeon, Joel Cervantes, doing his best to appear calm. Again Kennedy attempted to respond. It looked as if he was trying to force his brain to work, like someone with a sore throat who bears down to swallow.
Meanwhile, the surgeon’s mind kept circling back to the same uneasy thought: “I shouldn’t have done this.”
When Kennedy had arrived at the airport in Belize City a few days earlier, he had been lucid and precise, a 66-year-old with the stiff, authoritative good looks of a TV doctor. There had been nothing wrong with him, no medical need for Cervantes to open his skull. But Kennedy wanted brain surgery, and he was willing to pay $30,000 to have it done.
Kennedy was himself once a famous neurologist. In the late 1990s he made global headlines for implanting several wire electrodes in the brain of a paralyzed man and then teaching the locked-in patient to control a computer cursor with his mind. Kennedy called his patient the world’s “first cyborg,” and the press hailed his feat as the first time a person had ever communicated through a brain-computer interface. From then on, Kennedy dedicated his life to the dream of building more and better cyborgs and developing a way to fully digitize a person’s thoughts.
Now it was the summer of 2014, and Kennedy had decided that the only way to advance his project was to make it personal. For his next breakthrough, he would tap into a healthy human brain. His own.
Hence Kennedy’s trip to Belize for surgery. A local orange farmer and former nightclub owner, Paul Powton, had managed the logistics of Kennedy’s operation, and Cervantes—Belize’s first native-born neurosurgeon—wielded the scalpel. Powton and Cervantes were the founders of Quality of Life Surgery, a medical tourism clinic that treats chronic pain and spinal disorders and also specializes these days in tummy tucks, nose jobs, manboob reductions, and other medical enhancements.
At first the procedure that Kennedy hired Cervantes to perform—the implantation of a set of glass-and-gold-wire electrodes beneath the surface of his own brain—seemed to go quite well. There wasn’t much bleeding during the surgery. But his recovery was fraught with problems. Two days in, Kennedy was sitting on his bed when, all of a sudden, his jaw began to grind and chatter, and one of his hands began to shake. Powton worried that the seizure would break Kennedy’s teeth.
His language problems persisted as well. “He wasn’t making sense anymore,” Powton says. “He kept apologizing, ‘Sorry, sorry,’ because he couldn’t say anything else.” Kennedy could still utter syllables and a few scattered words, but he seemed to have lost the glue that bound them into phrases and sentences. When Kennedy grabbed a pen and tried to write a message, it came out as random letters scrawled on a page.
At first Powton had been impressed by what he called Kennedy’s Indiana Jones approach to science: tromping off to Belize, breaking the standard rules of research, gambling with his own mind. Yet now here he was, apparently locked in. “I thought we had damaged him for life,” Powton says. “I was like, what have we done?”
Of course, the Irish-born American doctor knew the risks far better than Powton and Cervantes did. After all, Kennedy had invented those glass-and-gold electrodes and overseen their implantation in almost a half dozen other people. So the question wasn’t what Powton and Cervantes had done to Kennedy—but what Phil Kennedy had done to himself.
FOR ABOUT AS long as there have been computers, there have been people trying to figure out a way to control them with our minds. In 1963 a scientist at Oxford University reported that he had figured out how to use human brain waves to control a simple slide projector. Around the same time, a Spanish neuroscientist at Yale University, José Delgado, grabbed headlines with a grand demonstration at a bullring in Córdoba, Spain. Delgado had invented a device he called a stimoceiver—a radio-controlled brain implant that could pick up neural signals and deliver tiny shocks to the cortex. When Delgado stepped into the ring, he flashed a red cape to incite the bull to charge. As the animal drew close, Delgado pressed two buttons on his radio transmitter: The first triggered the bull’s caudate nucleus and slowed the animal to a halt; the second made it turn and trot off toward a wall.
Delgado dreamed of using his electrodes to tap directly into human thoughts: to read them, edit them, improve them. “The human race is at an evolutionary turning point. We’re very close to having the power to construct our own mental functions,” he told The New York Times in 1970, after trying out his implants on mentally ill human subjects. “The question is, what sort of humans would we like, ideally, to construct?”
Not surprisingly, Delgado’s work made a lot of people nervous. And in the years that followed, his program faded, beset by controversy, starved of research funding, and stymied by the complexities of the brain, which was not as susceptible to simple hot-wiring as Delgado had imagined.
In the meantime, scientists with more modest agendas—who wanted simply to decipher the brain’s signals, rather than to grab civilization by the neurons—continued putting wires in the heads of laboratory animals. By the 1980s neuroscientists had figured out that if you use an implant to record signals from groups of cells in, say, the motor cortex of a monkey, and then you average all their firings together, you can figure out where the monkey means to move its limb—a finding many regarded as the first major step toward developing brain-controlled prostheses for human patients.
But the traditional brain electrode implants used in much of this research had a major drawback: The signals they picked up were notoriously unstable. Because the brain is a jellylike medium, cells sometimes drift out of range while they’re being recorded or end up dying from the trauma of colliding with a pointy piece of metal. Eventually electrodes can get so caked with scar tissue that their signals fade completely.
Phil Kennedy’s breakthrough—the one that would define his career in neuroscience and ultimately set him on a path to an operating table in Belize—started out as a way to solve this basic bioengineering problem. His idea was to pull the brain inside the electrode so the electrode would stay safely anchored inside the brain. To do this, he affixed the tips of some Teflon-coated gold wires inside a hollow glass cone. In the same tiny space, he inserted another crucial component: a thin slice of sciatic nerve. This crumb of biomaterial would serve to fertilize the nearby neural tissue, enticing microscopic arms from local cells to unfurl into the cone. Instead of plunging a naked wire into the cortex, Kennedy would coax nerve cells to weave their tendriled growths around the implant, locking it in place like a trellis ensnarled in ivy. (For human subjects he would replace the sciatic nerve with a chemical cocktail known to stimulate neural growth.)
The glass cone design seemed to offer an incredible benefit. Now researchers could leave their wires in situ for long stretches of time. Instead of catching snippets of the brain’s activity during single sessions in the lab, they could tune in to lifelong soundtracks of the brain’s electrical chatter.
Kennedy called his invention the neurotrophic electrode. Soon after he came up with it, he quit his academic post at Georgia Tech and started up a biotech company called Neural Signals. In 1996, after years of animal testing, Neural Signals received approval from the FDA to implant Kennedy’s cone electrodes in human patients, as a possible lifeline for people who had no other way to move or speak. And in 1998, Kennedy and his medical collaborator, Emory University neurosurgeon Roy Bakay, took on the patient who would make them scientific celebrities.
Johnny Ray was a 52-year-old drywall contractor and Vietnam veteran who had suffered a stroke at the base of his brain. The injury had left him on a ventilator, stuck in bed, and paralyzed except for slight twitchings of his face and shoulder. He could answer simple questions by blinking twice for “yes” and once for “no.”
Since Ray’s brain had no way to pass its signals down into his muscles, Kennedy tried to wiretap Ray’s head to help him communicate. Kennedy and Bakay placed electrodes in Ray’s primary motor cortex, the patch of tissue that controls basic voluntary movements. (They found the perfect spot by first putting Ray into an MRI machine and asking him to imagine moving his hand. Then they put the implant on the spot that lit up most brightly in his fMRI scans.) Once the cones were in place, Kennedy hooked them up to a radio transmitter implanted on top of Ray’s skull, just beneath the scalp.
Three times a week, Kennedy worked with Ray, trying to decode the waves from his motor cortex and then turn them into actions. As time went by, Ray learned to modulate the signals from his implant just by thinking. When Kennedy hooked him up to a computer, he was able to use those modulations to control a cursor on the screen (albeit only along a line from left to right). Then he’d twitch his shoulder to trigger a mouseclick. With this setup, Ray could pick out letters from an onscreen keyboard and very slowly spell out words.
“This is right on the cutting edge, it’s Star Wars stuff,” Bakay told an audience of fellow neurosurgeons in October 1998. A few weeks later, Kennedy presented their results at the annual conference of the Society for Neuroscience. That was enough to send the Amazing Story of Johnny Ray—once locked in, now typing with his mind—into newspapers all around the country and the world. That December both Bakay and Kennedy were guests on Good Morning America. In January 1999, news of their experiment appeared in The Washington Post. “As Philip R. Kennedy, physician and inventor, prepares a paralyzed man to operate a computer with his thoughts,” the article began, “it briefly seems possible a historic scene is unfolding in this hospital room and that Kennedy might be a new Alexander Graham Bell.”
IN THE AFTERMATH of his success with Johnny Ray, Kennedy seemed to be on the verge of something big. But when he and Bakay put brain implants in two more locked-in patients in 1999 and 2002, their cases didn’t push the project forward. (One patient’s incision didn’t close and the implant had to be removed; the other patient’s disease progressed so rapidly as to make Kennedy’s neural recordings useless.) Ray himself died from a brain aneurysm in the fall of 2002.
Meanwhile, other labs were making progress with brain-controlled prostheses, but they were using different equipment—usually small tabs, measuring a couple of millimeters square, with dozens of naked wire leads protruding down into the brain. In the format wars of the tiny neural-implants field, Kennedy’s glass-and-cone electrodes were looking more and more like Betamax: a viable, promising technology that ultimately didn’t take hold.
It wasn’t just hardware that set Kennedy apart from the other scientists working on brain-computer interfaces. Most of his colleagues were focused on a single type of neurally controlled prosthesis, the kind the Pentagon liked to fund through Darpa: an implant that would help a patient (or a wounded veteran) use prosthetic limbs. By 2003 a lab at Arizona State University had put a set of implants inside a monkey that allowed the animal to bring a piece of orange to its mouth with a mind-controlled robotic arm. Some years later researchers at Brown University reported that two paralyzed patients had learned to use implants to control robot arms with such precision that one could take a swig of coffee from a bottle.
But Kennedy was less interested in robot arms than in human voices. Ray’s mental cursor showed that locked-in patients could share their thoughts through a computer, even if those thoughts did dribble out like tar pitch at three characters per minute. What if Kennedy could build a brain-computer interface that flowed as smoothly as a healthy person’s speech?
In many ways, Kennedy had taken on the far greater challenge. Human speech is immensely more complicated than any movement of a limb. What seems to us a basic action—formulating words—requires the coordinated contraction and release of more than 100 different muscles, ranging from the diaphragm to those of the tongue and lips. To build a working speech prosthesis of the kind Kennedy imagined, a scientist would have to figure out a way to read all the elaborate orchestration of vocal language from the output of a handful of electrodes.
So Kennedy tried something new in 2004, when he put his implants in the brain of one last locked-in patient, a young man named Erik Ramsey, who had been in a car accident and suffered a brain stem stroke like Johnny Ray’s. This time Kennedy and Bakay did not place the cone electrodes in the part of the motor cortex that controls the arms and hands. They pushed the wires farther down a strip of brain tissue that drapes along the sides of the cerebrum like a headband. At the bottom of this region lies a patch of neurons that sends signals to the muscles of the lips and jaw and tongue and larynx. That’s where Ramsey got his implant, 6 millimeters deep.
Using this device, Kennedy taught Ramsey to produce simple vowel sounds through a synthesizer. But Kennedy had no way of knowing how Ramsey really felt or what exactly was going on in his head. Ramsey could respond to yes-no questions by moving his eyes up or down, but this method faltered because Ramsey had eye problems. Nor was there any way for Kennedy to corroborate his language trials. He’d asked Ramsey to imagine words while he recorded signals from Ramsey’s brain—but of course Kennedy had no way of knowing whether Ramsey really “said” the words in silence.
Ramsey’s health declined, as did the electronics for the implant in his head. As the years went by, Kennedy’s research program suffered too: His grants were not renewed; he had to let his engineers and lab techs go; his partner, Bakay, died. Now Kennedy worked alone or with temporary hired help. (He still spent business hours treating patients at his neurology clinic.) He felt sure he would make another breakthrough if he could just find another patient—ideally someone who could speak out loud, at least at first. By testing his implant on, say, someone in the early stages of a neurodegenerative disease like ALS, he’d have the chance to record from neurons while the person talked. That way, he could figure out the correspondence between each specific sound and neural cue. He’d have the time to train his speech prosthesis—to refine its algorithm for decoding brain activity.
But before Kennedy could find his ALS patient, the FDA revoked its approval for his implants. Under new rules, unless Kennedy could demonstrate that they were safe and sterile—a requirement that would itself require funding that he didn’t have—he says he was banned from using his electrodes on any more human subjects.
But Kennedy’s ambition didn’t dim; if anything, it overflowed. In the fall of 2012, he self-published a science fiction novel called 2051, which told the story of Alpha, an Irish-born neural electrode pioneer like Kennedy who lived, at the age of 107, as the champion and exemplar of his own technology: a brain wired up inside a 2-foot-tall life-support robot. The novel provided a kind of outline for Kennedy’s dreams: His electrodes wouldn’t simply be a tool for helping locked-in patients to communicate but would also be the engine of an enhanced and cybernetic future in which people live as minds in metal shells.
By the time he published his novel, Kennedy knew what his next move would be. The man who had become famous for implanting the very first brain-machine communication interface inside a human patient would once again do something that had never been done before. He had no other choices left. “What the hell,” he thought. “I’ll just do it on myself.”
A FEW DAYS after the operation in Belize, Powton paid one of his daily visits to the guesthouse where Kennedy was convalescing, a bright white villa a block away from the Caribbean. Kennedy’s recovery had continued to go poorly: The more effort he put into talking, the more he seemed to get locked up. And no one from the US, it became clear, was coming to take the doctor off Powton and Cervantes’ hands. When Powton called Kennedy’s fiancée and told her about the complications, she didn’t express much sympathy. “I tried stopping him, but he wouldn’t listen,” she said.
On this particular visit, though, things started to look up. It was a hot day, and Powton brought Kennedy a lime juice. When the two men went out into the garden, Kennedy tilted back his head and let out an easy and contented sigh. “It feels good,” he blurted after taking a sip.
Researcher as Human Guinea Pig
In 2014, Phil Kennedy hired a neurosurgeon in Belize to implant several electrodes in his brain and then insert a set of electronic components beneath his scalp. Back at home, Kennedy used this system to record his own brain signals in a months-long battery of experiments. His goal: Crack the neural code of human speech.
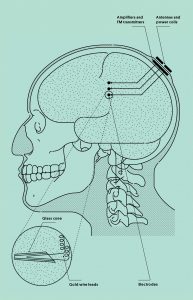
After that, Kennedy still had trouble finding words for things—he might look at a pencil and call it a pen—but his fluency improved. Once Cervantes felt his client had gotten halfway back to normal, he cleared him to go home. His early fears of having damaged Kennedy for life turned out to be unfounded; the language loss that left his patient briefly locked in was just a symptom of postoperative brain swelling. With that under control, he would be fine.
By the time Kennedy was back at his office seeing patients just a few days later, the clearest remaining indications of his Central American adventure were some lingering pronunciation problems and the sight of his shaved and bandaged head, which he sometimes hid beneath a multicolored Belizean hat. For the next several months, Kennedy stayed on anti-seizure medications as he waited for his neurons to grow inside the three cone electrodes in his skull.
Then, in October that same year, Kennedy flew back to Belize for a second surgery, this time to have a power coil and radio transceiver connected to the wires protruding from his brain. That surgery went fine, though both Powton and Cervantes were nonplussed at the components that Kennedy wanted tucked under his scalp. “I was a little surprised they were so big,” Powton says. The electronics had a clunky, retro look to them. Powton, who tinkers with drones in his spare time, was mystified that anyone would sew such an old-fangled gizmo inside his head: “I was like, ‘Haven’t you heard of microelectronics, dude?’ ”
Kennedy began the data-gathering phase of his grand self-experiment as soon as he returned home from Belize for the second time. The week before Thanksgiving, he went into his lab and balanced a magnetic power coil and receiver on his head. Then he started to record his brain activity as he said different phrases out loud and to himself—things like “I think she finds the zoo fun” and “The joy of a job makes a boy say wow”—while tapping a button to help sync his words with his neural traces, much like the way a filmmaker’s clapper board syncs picture and sound.
Over the next seven weeks, he spent most days seeing patients from 8 am until 3:30 pm and then used the evenings after work to run through his self-administered battery of tests. In his laboratory notes he is listed as Subject PK, as if to anonymize himself. His notes show that he went into the lab on Thanksgiving and on Christmas Eve.
The experiment didn’t last as long as he would have liked. The incision in his scalp never fully closed over the bulky mound of his electronics. After having had the full implant in his head for a total of just 88 days, Kennedy went back under the knife. But this time he didn’t bother going to Belize: A surgery to safeguard his health needed no approval from the FDA and would be covered by his regular insurance.
On January 13, 2015, a local surgeon opened up Kennedy’s scalp, snipped the wires coming from his brain, and removed the power coil and transceiver. He didn’t try to dig around in Kennedy’s cortex for the tips of the three glass cone electrodes that were embedded there. It was safer to leave those where they lay, enmeshed in Kennedy’s brain tissue, for the rest of his life.
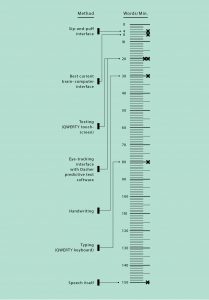
Loss for Words
Yes, it’s possible to communicate directly via your brain waves. But it’s excruciatingly slow. Other substitutes for speech get the job done faster.
KENNEDY’S LAB SITS in a leafy office park on the outskirts of Atlanta, in a yellow clapboard house. A shingle hanging out front identifies Suite B as the home of the Neural Signals Lab. When I meet Kennedy there one day in May 2015, he’s dressed in a tweed jacket and a blue-flecked tie, and his hair is neatly parted and brushed back from his forehead in a way that reveals a small depression in his left temple. “That’s when he was putting the electronics in,” Kennedy says with a slight Irish accent. “The retractor pulled on a branch of the nerve that went to my temporalis muscle. I can’t lift this eyebrow.” Indeed, I notice that the operation has left his handsome face with an asymmetric droop.
Kennedy agrees to show me the video of his first surgery in Belize, which has been saved to an old-fashioned CD-ROM. As I mentally prepare myself to see the exposed brain of the man standing next to me, Kennedy places the disc into the drive of a desktop computer running Windows 95. It responds with an awful grinding noise, like someone slowly sharpening a knife.
The disc takes a long time to load—so long that we have time to launch into a conversation about his highly unconventional research plan. “Scientists have to be individuals,” he says. “You can’t do science by committee.” As he goes on to talk about how the US too was built by individuals and not committees, the disc drive’s grunting takes on the timbre of a wagon rolling down a rocky trail: ga-chugga-chug, ga-chugga-chug. “Come on, machine!” he says, interrupting his train of thought as he clicks impatiently at some icons on the screen. “Oh for heaven’s sake, I just have inserted the disc!”
“I think people overrate brain surgery as being so terribly dangerous,” he goes on. “Brain surgery is not that difficult.” Ga-chugga-chug, ga-chugga-chug, ga-chugga-chug. “If you’ve got something to do scientifically, you just have to go and do it and not listen to naysayers.”
At last a video player window opens on the PC, revealing an image of Kennedy’s skull, his scalp pulled away from it with clamps. The grunting of the disc drive is replaced by the eerie, squeaky sound of metal bit on bone. “Oh, so they’re still drilling my poor head,” he says as we watch his craniotomy begin to play out onscreen.
“Just helping ALS patients and locked-in patients is one thing, but that’s not where we stop,” Kennedy says, moving on to the big picture. “The first goal is to get the speech restored. The second goal is to restore movement, and a lot of people are working on that—that’ll happen, they just need better electrodes. And the third goal would then be to start enhancing normal humans.”
He clicks the video ahead, to another clip in which we see his brain exposed—a glistening patch of tissue with blood vessels crawling all along the top. Cervantes pokes an electrode down into Kennedy’s neural jelly and starts tugging at the wire. Every so often a blue-gloved hand pauses to dab the cortex with a Gelfoam to stanch a plume of blood.
“Your brain will be infinitely more powerful than the brains we have now,” Kennedy continues, as his brain pulsates onscreen. “We’re going to extract our brains and connect them to small computers that will do everything for us, and the brains will live on.”
“You’re excited for that to happen?” I ask.
“Pshaw, yeah, oh my God,” he says. “This is how we’re evolving.”
Sitting there in Kennedy’s office, staring at his old computer monitor, I’m not so sure I agree. It seems like technology always finds new and better ways to disappoint us, even as it grows more advanced every year. My smartphone can build words and sentences from my sloppy finger-swipes. But I still curse at its mistakes. (Damn you, autocorrect!) I know that, around the corner, technology far better than Kennedy’s juddering computer, his clunky electronics, and my Google Nexus 5 phone is on its way. But will people really want to entrust their brains to it?
On the screen, Cervantes jabs another wire through Kennedy’s cortex. “The surgeon is very good, actually, a very nice pair of hands,” Kennedy said when we first started watching the video. But now he deviates from our discussion about evolution to bark orders at the screen, like a sports fan in front of a TV. “No, don’t do that, don’t lift it up,” Kennedy says to the pair of hands operating on his brain. “It shouldn’t go in at that angle,” he explains to me before turning back to the computer. “Push it in more than that!” he says. “OK, that’s plenty, that’s plenty. Don’t push anymore!”
THESE DAYS, INVASIVE brain implants have been going out of style. The major funders of neural prosthesis research favor an approach that involves laying a flat grid of electrodes, 8 by 8 or 16 by 16 of them, across the naked surface of the brain. This method, called electrocorticography, or ECoG, provides a more blurred-out, impressionistic measure of activity than Kennedy’s: Instead of tuning to the voices of single neurons, it listens to a bigger chorus—or, I suppose, committee—of them, as many as hundreds of thousands of neurons at a time.
Proponents of ECoG argue that these choral traces can convey enough information for a computer to decode the brain’s intent—even what words or syllables a person means to say. Some smearing of the data might even be a boon: You don’t want to fixate on a single wonky violinist when it takes a symphony of neurons to move your vocal cords and lips and tongue. The ECoG grid can also safely stay in place under the skull for a long time, perhaps even longer than Kennedy’s cone electrodes. “We don’t really know what the limits are, but it’s definitely years or decades,” says Edward Chang, a surgeon and neurophysiologist at UC San Francisco, who has become one of the leading figures in the field and who is working on a speech prosthesis of his own.
Last summer, as Kennedy was gathering his data to present it at the 2015 meeting of the Society for Neuroscience, another lab published a new procedure for using computers and cranial implants to decode human speech. Called Brain-to-Text, it was developed at the Wadsworth Center in New York in collaboration with researchers in Germany and the Albany Medical Center, and it was tested on seven epileptic patients with implanted ECoG grids. Each subject was asked to read aloud—sections of the Gettysburg Address, the story of Humpty Dumpty, John F. Kennedy’s inaugural, and an anonymous piece of fan fiction related to the TV show Charmed—while their neural data was recorded. Then the researchers used the ECoG traces to train software for converting neural data into speech sounds and fed its output into a predictive language model—a piece of software that works a bit like the speech-to-text engine on your phone—that could guess which words were coming based on what had come before.
Incredibly, the system kind of worked. The computer spat out snippets of text that bore more than a passing resemblance to Humpty Dumpty, Charmed fan fiction, and the rest. “We got a relationship,” says Gerwin Schalk, an ECoG expert and coauthor of the study. “We showed that it reconstructed spoken text much better than chance.” Earlier speech prosthesis work had shown that individual vowel sounds and consonants could be decoded from the brain; now Schalk’s group had shown that it’s possible—though difficult and error-prone—to go from brain activity to fully spoken sentences.
But even Schalk admits that this was, at best, a proof of concept. It will be a long time before anyone starts sending fully formed thoughts to a computer, he says—and even longer before anyone finds it really useful. Think about speech-recognition software, which has been around for decades, Schalk says. “It was probably 80 percent accurate in 1980 or something, and 80 percent is a pretty remarkable achievement in terms of engineering. But it’s useless in the real world,” he says. “I still don’t use Siri, because it’s not good enough.”
In the meantime, there are far simpler and more functional ways to help people who have trouble speaking. If a patient can move a finger, he can type out messages in Morse code. If a patient can move her eyes, she can use eye-tracking software on a smartphone. “These devices are dirt cheap,” Schalk says. “Now you want to replace one of these with a $100,000 brain implant and get something that’s a little better than chance?”
I try to square this idea with all the stunning cyborg demonstrations that have made their way into the media over the years—people drinking coffee with robotic arms, people getting brain implants in Belize. The future always seems so near at hand, just as it did a half century ago when José Delgado stepped into that bullring. One day soon we’ll all be brains inside computers; one day soon our thoughts and feelings will be uploaded to the Internet; one day soon our mental states will be shared and data-mined. We can already see the outlines of this scary and amazing place just on the horizon—but the closer we get, the more it seems to fall back into the distance.
Kennedy, for one, has grown tired of this Zeno’s paradox of human progress; he has no patience for always getting halfway to the future. That’s why he adamantly pushes forward: to prepare us all for the world he wrote about in 2051, the one that Delgado believed was just around the corner.
When Kennedy finally did present the data that he’d gathered from himself—first at an Emory University symposium last May and then at the Society for Neuroscience conference in October—some of his colleagues were tentatively supportive. By taking on the risk himself, by working alone and out-of-pocket, Kennedy managed to create a sui generis record of language in the brain, Chang says: “It’s a very precious set of data, whether or not it will ultimately hold the secret for a speech prosthetic. It’s truly an extraordinary event.” Other colleagues found the story thrilling, even if they were somewhat baffled: In a field that is constantly hitting up against ethical roadblocks, this man they’d known for years, and always liked, had made a bold and unexpected bid to force brain research to its destiny. Still other scientists were simply aghast. “Some thought I was brave, some thought I was crazy,” Kennedy says.
In Georgia, I ask Kennedy if he’d ever do the experiment again. “On myself?” he says. “No. I shouldn’t do this again. I mean, certainly not on the same side.” He taps his temple, where the cone electrode tips are still lodged. Then, as if energized by the idea of putting implants on the other side of his brain, he launches into plans for making new electrodes and more sophisticated implants; for getting back the FDA’s approval for his work; for finding grants so that he can pay for everything.
“No, I shouldn’t do the other side,” he says finally. “Anyway, I don’t have the electronics for it. Ask me again when we’ve built them.” Here’s what I take from my time with Kennedy, and from his garbled answer: You can’t always plan your path into the future. Sometimes you have to build it first.
by Danie Enger For Wired
Be the first to post a message!